A container containing 5.00 L of gas presents a fascinating realm of exploration into the behavior of gases and their interactions with their surroundings. This discourse delves into the fundamental properties of gases, the units used to quantify their volume, the relationship between gas density and volume, the principles governing gas mixtures and partial pressures, and the Ideal Gas Law that elucidates the interplay between pressure, volume, temperature, and the quantity of gas.
Understanding the behavior of gases confined within a container is crucial in various scientific disciplines and technological applications. From scuba diving to industrial gas storage and transportation, the principles governing gases play a pivotal role in ensuring safety and efficiency.
Properties of a Gas Container
Gas containers are designed to hold gases under pressure. They come in various shapes and sizes, and their properties can vary depending on the specific application. Understanding the properties of gas containers is essential for safe and efficient use.
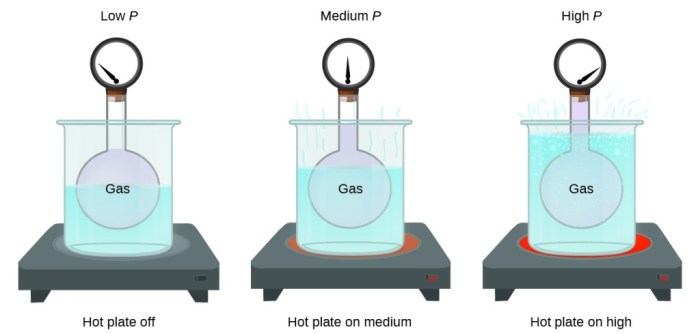
One important property of a gas container is its volume. The volume of a container determines the amount of gas it can hold. The relationship between volume and pressure in a gas container is described by Boyle’s Law, which states that the pressure of a gas is inversely proportional to its volume.
This means that as the volume of a gas container increases, the pressure of the gas decreases, and vice versa.
Another important property of a gas container is its ability to expand and contract. Gases have the property of expanding when heated and contracting when cooled. This means that the volume of a gas container will change depending on the temperature of the gas inside.
For example, if a gas container is filled with gas at room temperature and then heated, the gas will expand and the volume of the container will increase.
Applications of Gas Containers, A container containing 5.00 l of gas
Gas containers are used in a wide variety of applications, including:
- Storing and transporting gases for industrial and medical purposes
- Providing fuel for vehicles and appliances
- Creating pressure for various processes, such as welding and painting
The type of gas container used for a particular application will depend on the specific requirements of the application.
Units of Measurement for Gas Volume: A Container Containing 5.00 L Of Gas

The volume of a gas is a measure of the amount of space it occupies. The SI unit of volume is the liter (L). One liter is equal to 1000 cubic centimeters (cm 3) or 1 cubic decimeter (dm 3).
Other units of volume that are commonly used for gases include the milliliter (mL) and the cubic meter (m 3). One milliliter is equal to 1/1000 of a liter, and one cubic meter is equal to 1000 liters.
Conversion Between Volume Units
The following table summarizes the conversions between different volume units:
| Unit | Conversion to Liters |
|---|---|
| Milliliter (mL) | 1 mL = 0.001 L |
| Cubic Meter (m3) | 1 m3 = 1000 L |
Gas Density and Volume

Gas density, a crucial property, plays a significant role in various applications. It is defined as the mass of a gas per unit volume, providing insights into the compactness of the gas.
Calculating gas density is straightforward. Divide the mass of the gas by its volume. The formula is:
ρ = m/V
where:
- ρ is the gas density in kilograms per cubic meter (kg/m³)
- m is the mass of the gas in kilograms (kg)
- V is the volume of the gas in cubic meters (m³)
Factors such as temperature and pressure significantly influence gas density. Higher temperatures cause gas molecules to move faster, increasing the average distance between them and decreasing density. Conversely, higher pressures compress gas molecules, reducing the space between them and increasing density.
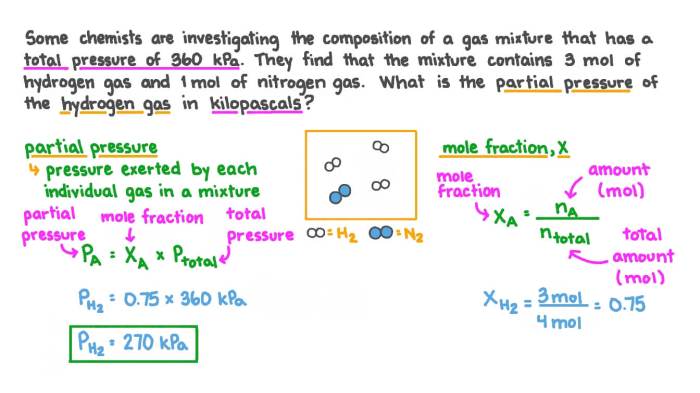
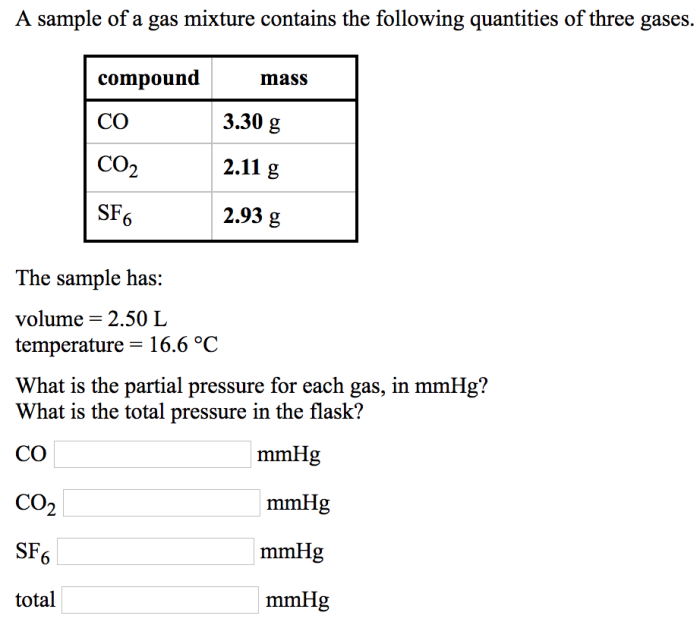
Gas Mixtures and Partial Pressures
A gas mixture is a combination of two or more gases that occupy the same space. Each gas in the mixture exerts its own pressure, called partial pressure. The partial pressure of a gas is the pressure it would exert if it occupied the entire volume alone.
Dalton’s Law of Partial Pressures
Dalton’s Law of Partial Pressures states that the total pressure of a gas mixture is equal to the sum of the partial pressures of each individual gas in the mixture. This law can be expressed mathematically as:
Ptotal= P 1+ P 2+ … + P n
where P totalis the total pressure of the mixture, and P 1, P 2, …, P nare the partial pressures of the individual gases.
Applications of Partial Pressures
Partial pressures are used in a variety of real-world applications, including scuba diving. When a scuba diver descends underwater, the pressure of the water increases. This increased pressure causes the partial pressure of nitrogen in the diver’s blood to increase.
If the diver ascends too quickly, the nitrogen can come out of solution and form bubbles in the blood, which can lead to decompression sickness.
Ideal Gas Law

The Ideal Gas Law is a mathematical equation that describes the relationship between the pressure, volume, temperature, and number of moles of a gas.
The Ideal Gas Law equation is:
PV = nRT
where:
- P is the pressure of the gas in pascals (Pa)
- V is the volume of the gas in cubic meters (m 3)
- n is the number of moles of gas in moles (mol)
- R is the ideal gas constant, which is equal to 8.314 J/(mol·K)
- T is the temperature of the gas in kelvins (K)
The Ideal Gas Law can be used to calculate any of the four variables (P, V, n, or T) if the other three are known.
The Ideal Gas Law is a good approximation for the behavior of many gases at low pressures and high temperatures. However, it does not account for all of the interactions between gas molecules, so it can be less accurate at high pressures and low temperatures.
Commonly Asked Questions
What is the relationship between volume and pressure in a gas container?
According to Boyle’s Law, the volume of a gas at constant temperature is inversely proportional to its pressure.
How is the density of a gas calculated?
Gas density is calculated by dividing the mass of the gas by its volume.
What is Dalton’s Law of Partial Pressures?
Dalton’s Law states that the total pressure exerted by a mixture of non-reacting gases is equal to the sum of the partial pressures of each individual gas.