Embark on an in-depth exploration of molarity practice problems worksheet answers, delving into the intricacies of solution concentration and its practical applications. This comprehensive guide provides a firm foundation in molarity calculations, empowering you to navigate the complexities of chemistry with confidence.
Throughout this discourse, we will unravel the concept of molarity, its significance in various scientific disciplines, and its indispensable role in quantitative analysis. Practice problems with step-by-step solutions will reinforce your understanding, while real-world examples will showcase the practical applications of molarity in diverse fields.
Definition of Molarity: Molarity Practice Problems Worksheet Answers
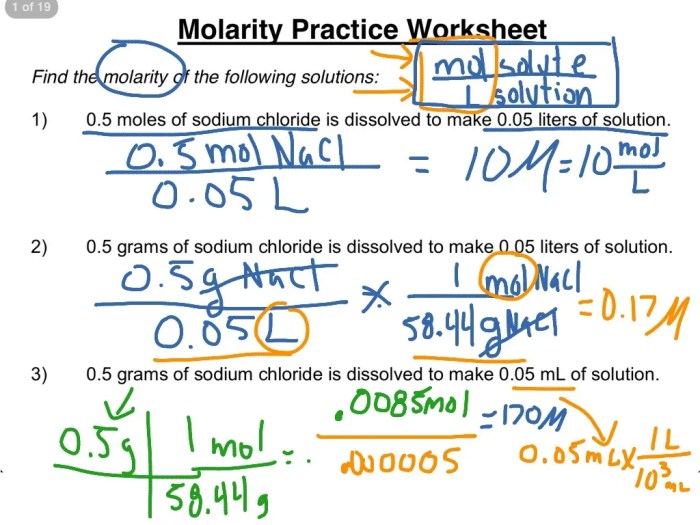
Molarity is a measure of the concentration of a solution, specifically the number of moles of solute per liter of solution. It is a fundamental concept in chemistry and is widely used in various fields such as analytical chemistry, biochemistry, and environmental science.
Molarity is expressed in units of moles per liter (mol/L), also known as molar (M). The formula for calculating molarity is:
Molarity = moles of solute / liters of solution
FAQ Explained
What is the formula for calculating molarity?
Molarity = moles of solute / liters of solution
How do I convert between molarity and molality?
Molarity = molality x density of solution
What are the applications of molarity in real-world scenarios?
Molarity is used in analytical chemistry, biochemistry, environmental science, and industrial processes, among others.