Conjugate acid base pairs worksheet answers – Delve into the realm of conjugate acid-base pairs worksheet answers, where the intricacies of chemical reactions unfold. This comprehensive guide unravels the concepts, questions, and solutions surrounding this fundamental aspect of chemistry, providing a clear understanding of acid-base relationships and their significance.
Within this detailed exploration, we delve into the definitions and examples of conjugate acid-base pairs, analyze the questions posed in the worksheet, and present a meticulously organized table of answers. The reasoning behind each answer is thoroughly explained, addressing any exceptions or special cases.
Additional examples and applications of conjugate acid-base pairs further solidify their importance in various chemical processes.
Conjugate Acid-Base Pairs: Conjugate Acid Base Pairs Worksheet Answers
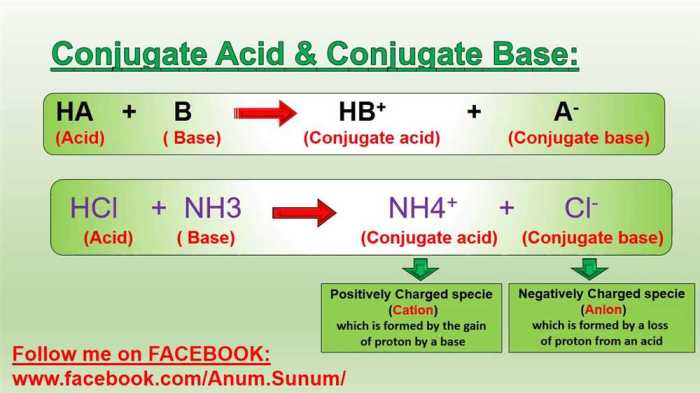
In chemistry, a conjugate acid-base pair is a pair of chemical species that differ by the transfer of a single proton (H +ion). The conjugate acid is the species that results from the addition of a proton to the base, and the conjugate base is the species that results from the removal of a proton from the acid.
Examples of Conjugate Acid-Base Pairs, Conjugate acid base pairs worksheet answers
- Water (H 2O) and hydronium ion (H 3O +)
- Ammonia (NH 3) and ammonium ion (NH 4+)
- Acetic acid (CH 3COOH) and acetate ion (CH 3COO –)
FAQ Compilation
What is the definition of a conjugate acid-base pair?
A conjugate acid-base pair consists of two species that differ by a single proton (H+ ion). When an acid donates a proton, its conjugate base is formed, and when a base accepts a proton, its conjugate acid is formed.
How do I identify the conjugate acid and base in a reaction?
The conjugate acid is the species that accepts a proton, while the conjugate base is the species that donates a proton. In an acid-base reaction, the acid donates a proton to the base, forming its conjugate base and the conjugate acid of the base.
What are some examples of conjugate acid-base pairs?
Common examples of conjugate acid-base pairs include: – H2O and OH- (water and hydroxide ion) – HCl and Cl- (hydrochloric acid and chloride ion) – NH3 and NH4+ (ammonia and ammonium ion)