Solubility and solubility curves worksheet – Embark on a journey into the realm of solubility and solubility curves, where we unravel the intricacies of substances and their interactions with solvents. This guide will illuminate the fundamental concepts, delve into the practical applications, and equip you with a comprehensive understanding of this fascinating topic.
Solubility, a measure of a substance’s ability to dissolve in a solvent, plays a crucial role in various scientific fields and industrial processes. Solubility curves, graphical representations of solubility data, provide valuable insights into the behavior of substances under different conditions.
Solubility
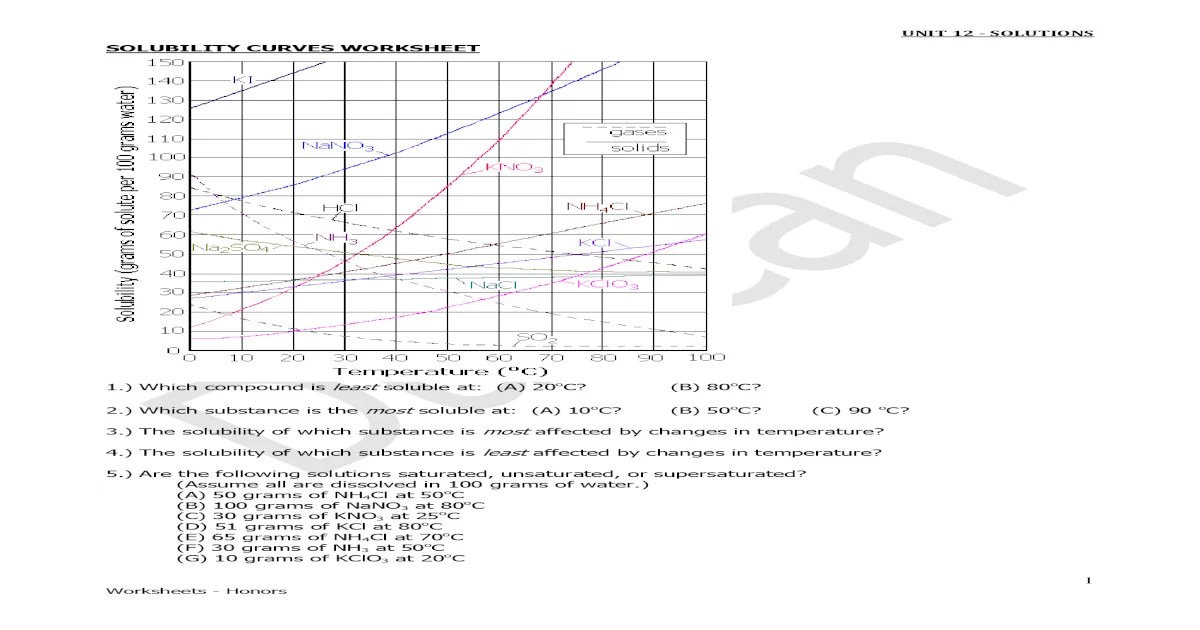
Solubility is the ability of a substance to dissolve in a solvent. The solubility of a substance is expressed in terms of the concentration of the substance in the solvent. The concentration of a solution can be expressed in terms of molarity, molality, or mass percentage.
The solubility of a substance is affected by a number of factors, including the temperature, pressure, and the nature of the solvent and the solute. In general, the solubility of a substance increases with increasing temperature and pressure. The solubility of a substance also increases with increasing polarity of the solvent and the solute.
Some substances are soluble in water, while others are insoluble. Soluble substances include sugar, salt, and alcohol. Insoluble substances include sand, oil, and wax.
Solubility Curves, Solubility and solubility curves worksheet
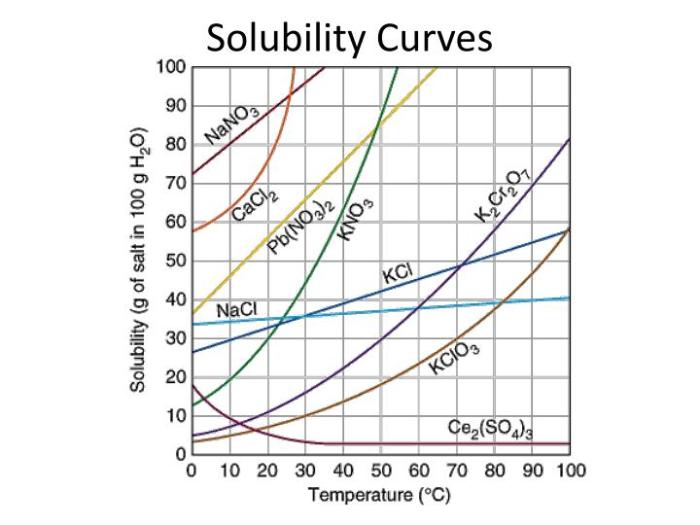
Solubility curves are graphs that show the relationship between the solubility of a substance and the temperature of the solvent. Solubility curves can be used to predict the solubility of a substance at a given temperature.
There are two types of solubility curves: linear and non-linear. Linear solubility curves indicate that the solubility of a substance increases linearly with increasing temperature. Non-linear solubility curves indicate that the solubility of a substance does not increase linearly with increasing temperature.
The shape of a solubility curve is affected by a number of factors, including the nature of the solvent and the solute, the presence of impurities, and the pressure.
Worksheet Activities
The following worksheet activities can be used to demonstrate the concept of solubility and solubility curves.
- Solubility of Sugar in Water:This activity demonstrates how the solubility of sugar in water changes with temperature. Students will dissolve sugar in water at different temperatures and plot the results on a graph.
- Solubility of Salt in Water:This activity demonstrates how the solubility of salt in water changes with temperature. Students will dissolve salt in water at different temperatures and plot the results on a graph.
- Solubility of Oil in Water:This activity demonstrates how the solubility of oil in water changes with temperature. Students will mix oil and water at different temperatures and observe the results.
Solubility Curve Examples
The following table shows solubility curves for different substances.
| Substance | Solubility Curve |
|---|---|
| Sugar | Linear |
| Salt | Linear |
| Oil | Non-linear |
The solubility of sugar and salt in water increases linearly with increasing temperature. The solubility of oil in water does not increase linearly with increasing temperature.
Applications of Solubility Curves
Solubility curves have a number of practical applications in industry and research.
- Solubility curves can be used to predict the solubility of a substance at a given temperature.
- Solubility curves can be used to design processes for the separation of substances.
- Solubility curves can be used to study the interactions between different substances.
FAQ: Solubility And Solubility Curves Worksheet
What factors affect the solubility of a substance?
Temperature, pressure, solvent polarity, and the nature of the solute
How can solubility curves be used to predict the solubility of a substance?
By extrapolating the curve to the desired temperature or pressure
What are some practical applications of solubility curves?
Designing crystallization processes, optimizing extraction techniques, and understanding environmental phenomena