Phase change graph worksheet answers – Unlock the secrets of phase change graphs with our comprehensive worksheet answers. Dive into the intricacies of phase transitions, unraveling the mysteries behind melting, freezing, vaporization, and condensation. Prepare to embark on a journey that will transform your understanding of these fundamental physical processes.
Our expertly crafted guide provides a roadmap for interpreting phase change graphs, empowering you to decipher the temperature and time dynamics of each transition. Discover the significance of slope and plateaus, and delve into the practical applications of phase change graphs in engineering, chemistry, and beyond.
Understanding Phase Change Graphs
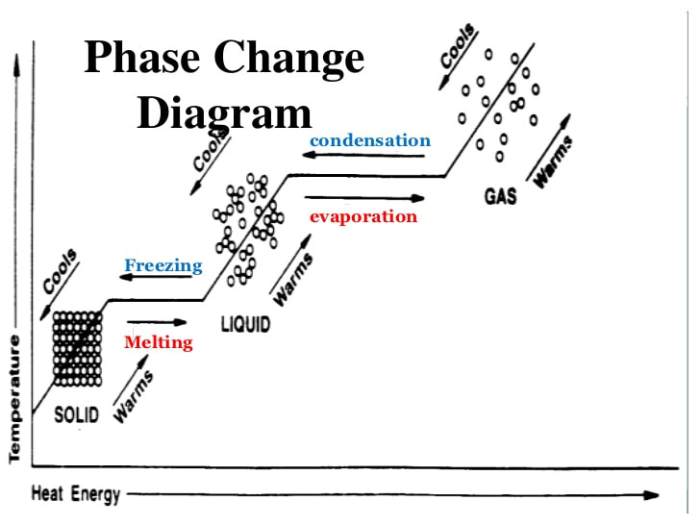
Phase changes are physical processes that involve the transformation of a substance from one state to another. The three main phase changes are:
-
-*Melting
Solid to liquid
-*Freezing
Liquid to solid
-*Vaporization
Liquid to gas
-*Condensation
Gas to liquid
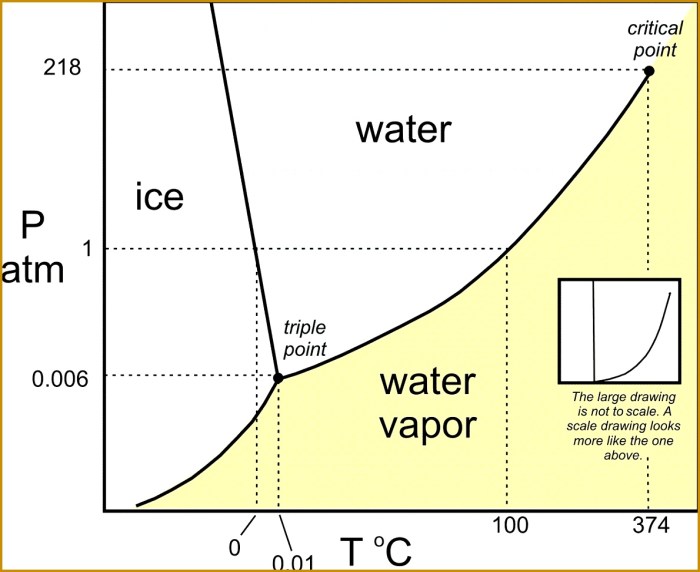
A phase change graph is a graphical representation of the temperature and pressure changes that occur during a phase change. The x-axis of the graph typically represents temperature, while the y-axis represents pressure.The slope of a phase change graph indicates the rate of temperature change with respect to pressure.
A positive slope indicates that the temperature is increasing with increasing pressure, while a negative slope indicates that the temperature is decreasing with increasing pressure.The plateaus in a phase change graph represent the temperatures at which the phase change occurs.
During a phase change, the temperature remains constant while the pressure changes.Phase change graphs are useful for understanding the thermodynamics of phase changes and for predicting the conditions under which a phase change will occur.
Types of Phase Changes
Phase changes are processes in which a substance changes from one state of matter to another. The three main states of matter are solid, liquid, and gas. Each phase change has unique characteristics that can be represented on a graph.
Melting
Melting is the process in which a solid changes to a liquid. This occurs when the temperature of the solid is raised to its melting point, at which point the solid particles gain enough energy to overcome the attractive forces holding them in a fixed position.
The melting point is the temperature at which the solid and liquid phases coexist in equilibrium.
Freezing
Freezing is the reverse of melting, in which a liquid changes to a solid. This occurs when the temperature of the liquid is lowered to its freezing point, at which point the liquid particles lose energy and slow down enough to form a solid structure.
The freezing point is the temperature at which the liquid and solid phases coexist in equilibrium.
Vaporization
Vaporization is the process in which a liquid changes to a gas. This can occur in two ways: evaporation and boiling. Evaporation is the process in which liquid particles at the surface of a liquid gain enough energy to overcome the attractive forces holding them to the liquid and escape into the gas phase.
Boiling is the process in which vaporization occurs throughout the entire liquid, not just at the surface. The boiling point is the temperature at which the liquid and gas phases coexist in equilibrium.
Condensation
Condensation is the reverse of vaporization, in which a gas changes to a liquid. This occurs when the temperature of the gas is lowered to its condensation point, at which point the gas particles lose energy and slow down enough to form a liquid.
The condensation point is the temperature at which the gas and liquid phases coexist in equilibrium.
Interpreting Phase Change Graphs
Phase change graphs are a graphical representation of the temperature and time required for a substance to undergo a phase change. They can be used to determine the temperature at which a substance melts, freezes, boils, or condenses, as well as the time it takes for these processes to occur.
To interpret a phase change graph, first, locate the horizontal axis, which represents time, and the vertical axis, which represents temperature. The graph will typically show four distinct regions, corresponding to the solid, liquid, and gas phases of the substance.
Identifying Phase Changes
The phase changes are indicated by changes in the slope of the graph. When the slope is zero, the substance is not undergoing a phase change. When the slope is positive, the substance is gaining heat and its temperature is increasing.
When the slope is negative, the substance is losing heat and its temperature is decreasing.
The temperature at which a phase change occurs is indicated by the intersection of two lines on the graph. For example, the melting point of a substance is the temperature at which the solid and liquid lines intersect. The boiling point of a substance is the temperature at which the liquid and gas lines intersect.
Calculating Time and Temperature
The time required for a phase change to occur can be determined by measuring the horizontal distance between the two lines on the graph. For example, the time it takes for a substance to melt can be determined by measuring the horizontal distance between the solid and liquid lines.
The temperature at which a phase change occurs can be determined by measuring the vertical distance between the two lines on the graph. For example, the melting point of a substance can be determined by measuring the vertical distance between the solid and liquid lines.
Rate of Phase Change, Phase change graph worksheet answers
The rate of phase change is indicated by the slope of the graph. A steeper slope indicates a faster rate of phase change. For example, a steeper slope on the liquid-gas line indicates that the substance is boiling more rapidly.
Applications of Phase Change Graphs
Phase change graphs are valuable tools with diverse applications across various scientific and engineering fields. They provide insights into the behavior of substances undergoing phase changes and allow for the analysis and prediction of their properties.
Engineering
Phase change graphs are crucial in designing and optimizing thermal systems, such as heat exchangers, refrigeration systems, and power plants. By understanding the phase behavior of working fluids, engineers can determine the most efficient operating conditions and minimize energy losses.
Chemistry
In chemistry, phase change graphs are used to study the purity of substances. The melting point and boiling point of a pure substance are characteristic properties, and any deviations from these values can indicate impurities. Phase change graphs also aid in the identification of unknown substances by comparing their phase behavior with known compounds.
Meteorology
Phase change graphs are essential in meteorology for understanding and predicting weather patterns. They help meteorologists analyze the behavior of water in the atmosphere, including the formation of clouds, precipitation, and storms. By studying the phase changes of water vapor, they can forecast weather conditions and issue warnings for extreme events.
Answers to Common Questions: Phase Change Graph Worksheet Answers
What is a phase change graph?
A phase change graph is a graphical representation of the temperature and time changes that occur during a phase transition, such as melting, freezing, vaporization, or condensation.
How can I interpret the slope of a phase change graph?
The slope of a phase change graph indicates the rate of temperature change during the phase transition. A steeper slope represents a faster rate of temperature change.
What are some practical applications of phase change graphs?
Phase change graphs are used in various fields, including engineering, chemistry, and meteorology. They can be used to design heating and cooling systems, optimize chemical processes, and predict weather patterns.